
Technical
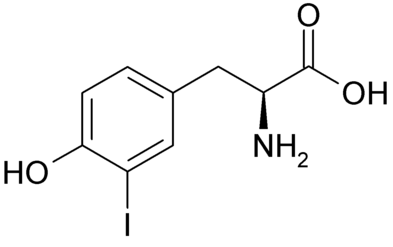
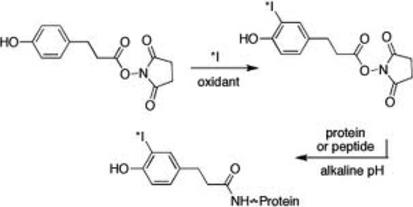
This labelling method has the advantage that it overcomes the problems of iodination damage associated with exposure of proteins to oxidising and reducing agents. There is minimal chemical damage to the protein and the labelling site is different from other labelling methods which is useful for proteins that lack suitable tyrosine residues for direct iodination. Using this conjugation method, it is possible to produce labelled enzyme preparations in which the activity is fully retained. It is also possible to produce iodinated tracers with a high retention of immunological activity from unstable proteins highly susceptible to damage from chloramine-T. This is of particular interest when considering iodination methods to be used for proteins that are to be used in Radio Immuno Assays (RIA).
Iodination yield
None
Immunological activity
–
Some possible causes
Low
Normal
Normal or impaired
Impaired
Normal or high
Impaired
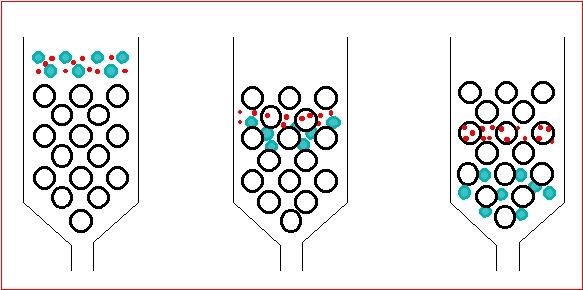
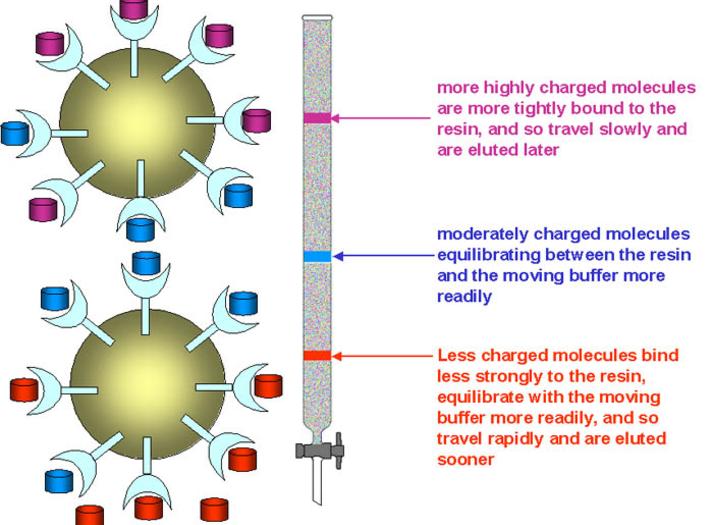
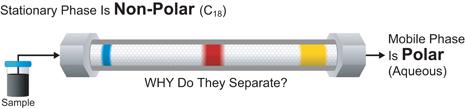
Affinity chromatography is particularly useful when labelling immunologically active materials and receptor ligands. The conditions required to remove labelled the ligand from the affinity material are usually fairly harsh and can damage the radioiodinated protein. Affinity chromatography does not remove or separate the unlabelled material. Ion-exchange chromatography which separates according to charge can be used to separate di-iodinated products and unlabelled material as well as lactoperoxidase but will not separate various multi-iodinated species or oxidised material. Reversed-phase HPLC can be used to separate various mono-iodinated materials from all the other reaction products and yield a pure product at maximum specific activity. The latter technique is ideally suited to small peptides (<8,000 mol.wt).
